Methoxycarbonylation of olefins catalyzed by palladium complexes bearing P,N-donor ligands†
Abstract
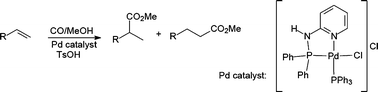
The methoxycarbonylation of
* Corresponding authors
a
Universidad de Chile, Facultad de Ciencias Químicas y Farmacéuticas, Departamento de Inorgánica y Analítica, Casilla 233 Santiago 1, Chile
E-mail:
paguirre@ciq.uchi le.cl
b Universidad de Santiago de Chile, Facultad de Química y Biología, Departamento de Química de los Materiales, Bernardo O'Higgins 3363, Santiago, Chile
c Departamento de Química Inorgánica, Instituto de Ciencia de Materiales de Aragón, Universidad de Zaragoza-CSIC, Zaragoza, Spain
d Servei Central d’Instrumentació Científica, Universitat Jaume I, Castelló de la Plana, Spain
e Departament de Química, Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain
The methoxycarbonylation of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
P. A. Aguirre, C. A. Lagos, S. A. Moya, C. Zúñiga, C. Vera-Oyarce, E. Sola, G. Peris and J. C. Bayón, Dalton Trans., 2007, 5419 DOI: 10.1039/B704615B
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
