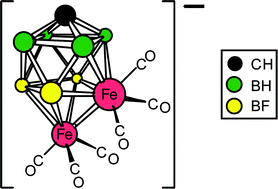
The ferracarborane [N(PPh3)2][6,6,6,10,10,10-(CO)6-closo-6,10,1-Fe2CB7H8] (2) reacts in CH2Cl2 with 3 molar equivalents of Ag[PF6] to yield the trifluoro-substituted species [N(PPh3)2][7,8,9-F3-6,6,6,10,10,10-(CO)6-closo-6,10,1-Fe2CB7H5] (3). Compound 3 undergoes structural rearrangement in toluene at reflux temperatures, forming [N(PPh3)2][8,9,10-F3-6,6,6,7,7,7-(CO)6-closo-6,7,1-Fe2CB7H5] (4). Alternatively, reaction of either 2 or 3 with a 10-fold excess of Ag[PF6] in CH2Cl2 forms two species: namely, [N(PPh3)2][2,7,9,10-F4-6,6,6,8,8,8-(CO)6-closo-6,8,1-Fe2CB7H4] (5), in which one further B–F substitution has occurred and the {Fe2CB7} cluster core has rearranged, plus a mono-iron co-product, [N(PPh3)2][3,8,9-F3-7,7,7-(CO)3-closo-7,1-FeCB7H5] (6) that is formed by polyhedral contraction. Treatment of 3 with [NO][BF4] in CH2Cl2 results in CO substitution at the 4-connected iron vertex [Fe(10)], producing the zwitterionic complex [7,8,9-F3-6,6,6,10,10-(CO)5-10-NO-closo-6,10,1-Fe2CB7H5] (7). Addition of Me3NO to a mixture of 3 and PEt3 in CH2Cl2 also results in CO substitution, forming the isomeric species [N(PPh3)2][7,8,9-F3-6,6,m,10,10-(CO)5-n-PEt3-closo-6,10,1-Fe2CB7H5] [m = 6, n = 10 (8a); m = 10, n = 6 (8b)] in a 5 : 1 ratio. Treatment of 8a with [NO][BF4] and then CNBut in CH2Cl2 allows further, successive CO substitution at Fe(10) to yield first a neutral, zwitterionic complex [7,8,9-F3-6,6,6,10-(CO)4-10-NO-10-PEt3-closo-6,10,1-Fe2CB7H5] (9) and then [7,8,9-F3-6,6,6-(CO)3-10-CNBut-10-NO-10-PEt3-closo-6,10,1-Fe2CB7H5] (10). The molecular structures of compounds 3, 4, 5, 6, and 10 have been established by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...