Activation of the C–I and C–OH bonds of 2-iodoethanol by gas phase silver cluster cations yields subvalent silver-iodide and -hydroxide cluster cations†‡
Abstract
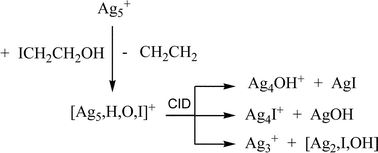
The gas phase ion–molecule reactions of silver cluster cations (Agn+) and silver hydride cluster cations (AgmH+) with 2-iodoethanol have been examined using multistage mass spectrometry experiments in a quadrupole ion trap mass spectrometer. These clusters exhibit size selective reactivity: Ag2H+, Ag3+, and Ag4H+ undergo sequential ligand addition only, while Ag5+ and Ag6H+ also promote both C–I and C–OH bond activation of 2-iodoethanol. Collision induced dissociation (CID) of Ag5HIO+, the product of C–I and C–OH bond activation by Ag5+, yielded Ag4OH+, Ag4I+ and Ag3+, consistent with a structure containing AgI and AgOH moieties. Ag6H+ promotes both C–I and C–OH bond activation of 2-iodoethanol to yield the metathesis product Ag6I+ as well as Ag6H2IO+. The metathesis product Ag6I+ also promotes C–I and C–OH bond activation.
DFT calculations were carried out to gain insights into the reaction of Ag5+ with ICH2CH2OH by calculating possible structures and their energies for the following species: (i) initial adducts of Ag5+ and ICH2CH2OH, (ii) the subsequent Ag5HIO+ product, (iii) CID products of Ag5HIO+. Potential adducts were probed by allowing ICH2CH2OH to bind in different ways (monodentate through I, monodentate through OH, bidentate) at different sites for two isomers of Ag5+: the global minimum “bowtie” structure, 1, and the higher energy trigonal bipyramidal isomer, 2. The following structural trends emerged: (i) ICH2CH2OH binds in a monodentate fashion to the silver core with little distortion, (ii) ICH2CH2OH binds to 1 in a bidentate fashion with some distortion to the silver core, and (iii) ICH2CH2OH binds to 2 and results in a significant distortion or rearrangement of the silver core. The DFT calculated minimum energy structure of Ag5HIO+ consists of an OH ligated to the face of a distorted trigonal bipyramid with I located at a vertex, while those for both Ag4X+ (X = OH, I) involve AgX bound to a Ag3+ core. The calculations also predict the following: (i) the ion–molecule reaction of Ag5+ and ICH2CH2OH to yield Ag5HIO+ is exothermic by 34.3 kcal mol−1, consistent with the fact that this reaction readily occurs under the near thermal experimental conditions, (ii) the lowest energy products for fragmentation of Ag5HIO+ arise from loss of AgI, consistent with this being the major pathway in the CID experiments.

 Please wait while we load your content...
Please wait while we load your content...