Metalloid group 14 cluster compounds: An introduction and perspectives to this novel group of cluster compounds†
Abstract
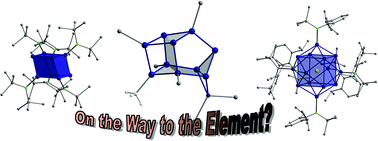
Metalloid cluster compounds of group 14 of the general formulae EnRm with n > m (E = Si, Ge, Sn and Pb, tetrel elements; R =
During the last years many different syntheses of such novel cluster compounds have been introduced, leading to several metalloid group 14 cluster compounds which exhibit new and unusual structure and bonding properties. In this tutorial review an account is given of the first steps in this novel field of group 14 chemistry. Special attention is focused on structural features and bonding properties.

 Please wait while we load your content...
Please wait while we load your content...