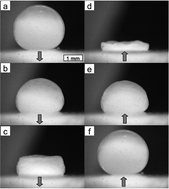
The mechanical response of particle-laden fluid interfaces is determined by measuring the internal pressures of particle-coated drops as a function of the drop volume. The particle monolayers undergoing compression–expansion cycles exhibit three distinct states: fluid state, jammed state, and buckled state. The P–V curves are compared to the surface pressure isotherms Π–A that are measured using a Langmuir trough and a Wilhelmy plate on a flat water–decane interface covered with the same particles. We find that in the fluid and jammed states, the water drop in decane can be described by the Young–Laplace equation. Therefore in these relatively low compression states, the bulk pressure measurements can be used to deduce the interfacial tension of the droplets and yield similar surface pressure isotherms to the ones measured with the Wilhelmy plate. In the buckled state, the internal pressure of the drop yields a zero value, which is consistent with the zero interfacial tension measured with the Wilhelmy plate. Moreover we find that the compressibility in the jammed state does not depend on the particle size.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...