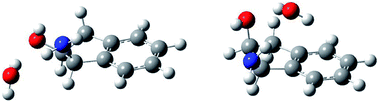
The role of conformational isomerism in molecular interaction has been studied using the example of jet-cooled complexes of (±)-cis-1-amino-indan-2-ol with water. The two formerly evidenced conformers of (±)-cis-1-amino-indan-2-ol easily form hydrates and dihydrates, which have been studied by means of laser-induced fluorescence and IR/UV double resonance spectroscopy, as well as ab initio calculations. All the 1 : 1 and 1 : 2 complexes with water evidenced in this work involve “ring” structures, in which the water monomer or dimer acts as an acceptor from the NH2 and a donor to the OH groups of (±)-cis-1-amino-indan-2-ol. However, the water lies externally to the indan frame in the hydrates of conformer I of (±)-cis-1-amino-indan-2-ol, which possesses axial NH2 and equatorial OH groups, and above it for the hydrates with the less stable conformer II, with equatorial NH2 and axial OH groups. Consequently, the different steric constraints which exist in the two conformers result in different hydrogen bond topologies, with an additional OH⋯π interaction for the hydrates of conformer II.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...