Chlorine–benzene complexes—the reliability of density functionals for non-covalent radical complexes†‡
Abstract
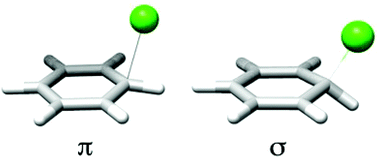
The structure of the chlorine atom–benzene complex has been a topic of significant controversy for more than 50 years. We have reexamined the structure of this complex with new density functional methods especially designed for non-covalent complexes, and compared the structures and energetics to those obtained using standard DFT and high accuracy composite methods. We find that the popular B3LYP functional fails to identify stationary points revealed by other functionals, and that the η1–σ complex appears to be more stable than the η1–π complex, contrary to other recent work, highlighting the careful selection of methods required in non-covalent radical systems.

 Please wait while we load your content...
Please wait while we load your content...