NMR studies of double proton transfer in hydrogen bonded cyclic N,N′-diarylformamidine dimers: conformational control, kinetic HH/HD/DD isotope effects and tunneling
Abstract
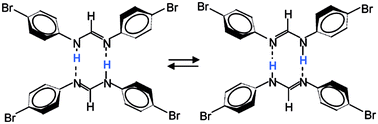
Using dynamic NMR spectroscopy, the kinetics of the degenerate double proton transfer in cyclic dimers of polycrystalline 15N,15N′-di-(4-bromophenyl)-formamidine (DBrFA) have been studied including the kinetic HH/HD/DD isotope effects in a wide temperature range. This transfer is controlled by intermolecular interactions, which in turn are controlled by the molecular conformation and hence the molecular structure. At low temperatures, rate constants were determined by line shape analysis of 15N NMR spectra obtained using cross-polarization (CP) and magic angle spinning (MAS). At higher temperatures, in the microsecond time scale, rate constants and kinetic isotope effects were obtained by a combination of longitudinal 15N and 2H relaxation measurements. 15N CPMAS line shape analysis was also employed to study the non-degenerate double proton transfer of polycrystalline 15N,15N′-diphenyl-formamidine (DPFA). The kinetic results are in excellent agreement with the kinetics of DPFA and 15N,15N′-di-(4-fluorophenyl)-formamidine (DFFA) studied previously for solutions in tetrahydrofuran. Two large HH/HD and HD/DD isotope effects are observed in the whole temperature range which indicates a concerted double proton transfer mechanism in the domain of the reaction energy surface. The Arrhenius curves are non-linear indicating a tunneling mechanism. Arrhenius curve simulations were performed using the Bell–Limbach tunneling model. The role of the phenyl group conformation and hydrogen bond compression on the barrier of the proton transfer is discussed.

 Please wait while we load your content...
Please wait while we load your content...