Theoretical analysis of the hydrogen bond of imidazolium C2–H with anions
Abstract
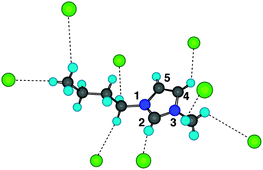
The intermolecular interaction energies of ion pairs of imidazolium-based
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions

 Please wait while we load your content...
Please wait while we load your content...