The products of the Cl atom initiated oxidation of diethyl ether (DEE) were investigated at atmospheric pressure over a range of temperatures (218–335 K) and O2 partial pressures (50–700 Torr), both in the presence and absence of NOx. The major products observed at 298 K and below were ethyl formate and ethyl acetate, which accounted for ≈60–80% of the reacted diethyl ether. In general, the yield of ethyl formate increased with increasing temperature, with decreasing O2 partial pressure, and upon addition of NO to the reaction mixtures. The product yield data show that thermal decomposition reaction (3), CH3CH2–O–CH(O˙)CH3 → CH3CH2–O–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + CH3, and reaction (6) with O2, CH3CH2–O–CH(O˙)CH3 + O2 → CH3CH2–O–C(
O + CH3, and reaction (6) with O2, CH3CH2–O–CH(O˙)CH3 + O2 → CH3CH2–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH3 + HO2 are competing fates of the CH3CH2–O–CH(O˙)CH3 radical, with a best estimate of k3/k6 ≈ 6.9 × 1024 exp(–3130/T). Thermal decomposition via C–H or C–O bond cleavage are at most minor contributors to the CH3CH2–O–CH(O˙)CH3 chemistry. The data also show that the CH3CH2–O–CH(O˙)CH3 radical is subject to a chemical activation effect. When produced from the exothermic reaction of the CH3CH2–O–CH(OO˙)CH3 radical with NO, prompt decomposition via both CH3- and probably H-elimination occur, with yields of about 40% and ≤15%, respectively. Finally, at temperatures slightly above ambient, evidence for a change in mechanism in the absence of NOx, possibly due to chemistry involving the peroxy radical CH3CH2–O–CH(OO˙)CH3, is presented.
O)CH3 + HO2 are competing fates of the CH3CH2–O–CH(O˙)CH3 radical, with a best estimate of k3/k6 ≈ 6.9 × 1024 exp(–3130/T). Thermal decomposition via C–H or C–O bond cleavage are at most minor contributors to the CH3CH2–O–CH(O˙)CH3 chemistry. The data also show that the CH3CH2–O–CH(O˙)CH3 radical is subject to a chemical activation effect. When produced from the exothermic reaction of the CH3CH2–O–CH(OO˙)CH3 radical with NO, prompt decomposition via both CH3- and probably H-elimination occur, with yields of about 40% and ≤15%, respectively. Finally, at temperatures slightly above ambient, evidence for a change in mechanism in the absence of NOx, possibly due to chemistry involving the peroxy radical CH3CH2–O–CH(OO˙)CH3, is presented.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + CH3, and reaction
O + CH3, and reaction ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)CH3 + HO2 are competing fates of the CH3CH2–O–CH(O˙)CH3 radical, with a best estimate of k3/k6 ≈ 6.9 × 1024 exp(–3130/T). Thermal decomposition via C–H or C–O bond cleavage are at most minor contributors to the CH3CH2–O–CH(O˙)CH3 chemistry. The data also show that the CH3CH2–O–CH(O˙)CH3 radical is subject to a chemical activation effect. When produced from the exothermic reaction of the CH3CH2–O–CH(OO˙)CH3 radical with
O)CH3 + HO2 are competing fates of the CH3CH2–O–CH(O˙)CH3 radical, with a best estimate of k3/k6 ≈ 6.9 × 1024 exp(–3130/T). Thermal decomposition via C–H or C–O bond cleavage are at most minor contributors to the CH3CH2–O–CH(O˙)CH3 chemistry. The data also show that the CH3CH2–O–CH(O˙)CH3 radical is subject to a chemical activation effect. When produced from the exothermic reaction of the CH3CH2–O–CH(OO˙)CH3 radical with 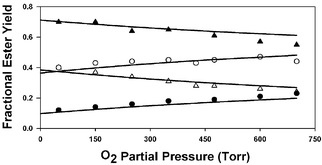

 Please wait while we load your content...
Please wait while we load your content...