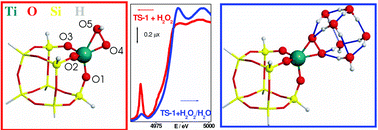
This work is intended to underline how the most-advanced experimental and theoretical physical chemistry tools must be used synergistically to understand the reactivity of Ti–silicalite-1 (TS-1) in an important number of low-temperature oxidation reactions with aqueous H2O2 as the oxidant. Literature results are carefully reviewed and accompanied with new, unpublished highlights of both experimental and computational origin. The first part of this work is devoted to a discussion of the defective nature of the material and to the synergic role played by Si vacancies and Ti insertion in the framework. A summary of the experimental Ti–K-edge EXAFS and XANES literature concerning the activated material in vacuo conditions is then presented and compared to the corresponding Ti geometries obtained by ab initio calculations. From such a comparison, the excellent agreement between experiment and theory is evident. A very good agreement is also obtained for the interaction with water and ammonia. For both H2O and NH3, the agreement is due to the possibility to perform experiments in which the probe molecule is dosed from the gas phase, thus allowing to reach the 1 : 1 (or 1 : 2) ratio between the adsorbing Ti sites and the adsorbed molecule. Then, interaction with hydrogen peroxide is discussed, underlining the problems faced in reaching a common view between experimental and theoretical results, owing to the difficulties both in performing experiments where anhydrous H2O2 is dosed on TS-1, and in taking into account the role played by the aqueous medium in the reactivity of Ti(IV) centres toward H2O2 using ab initio calculations. Only once such difficulties have been overcome, by increasing the complexities of both experiments and ab initio models, is a joint-view finally obtained. Where needed, comparison with other experimental results (X-ray and neutron diffraction, NMR, IR, Raman, UV-vis and resonant Raman) is made.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...