Phase field modeling of CH4hydrate conversion into CO2 hydrate in the presence of liquid CO2†
Abstract
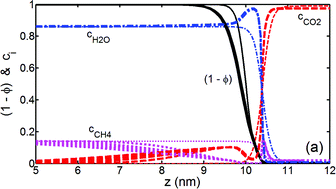
We present phase field simulations to estimate the conversion rate of CH4 hydrate to CO2 hydrate in the presence of liquid CO2 under conditions typical for underwater gas hydrate reservoirs. In the computations, all model parameters are evaluated from physical properties taken from experiment or molecular dynamics simulations. It has been found that

 Please wait while we load your content...
Please wait while we load your content...