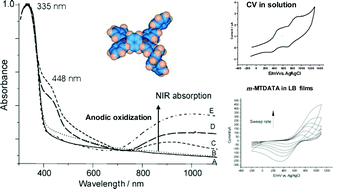
Here we report on the electroactivity properties of Langmuir–Blodgett (LB) films of the hole-transport molecule 4,4′,4″-tris[3-methylphenyl(phenyl)amino] triphenylamine (m-MTDATA). Fairly stable Langmuir films at the air–water interface are accomplished, despite the non-amphiphilic character of the molecule. The reflection-absorption infrared spectroscopy (RAIRS) and Fourier transform infrared (FT-IR) analysis revealed that the molecules arrange with no neat preferential orientation, in agreement with the amorphous glassy nature of this starburst molecule. However, there is a tendency of the molecules to organize in a more planar conformation due to the intermolecular stacking induced by the LB technique. On the other hand, the fundamental electrochemistry (by cyclic voltammetry, CV) of the films is also analyzed. The CV studies of both solution and films reveal that both the solid state and the electrolyte’s anions clearly affect the m-MTDATA’s electroactivity, exhibiting a unique and broad redox process instead of the two reversible oxidations observed in solution. The oxidization mechanism is discussed. Finally, the spectroelectrochemistry studies evidence that the oxidization of the films leads to new absorption bands, among which the emerging bands in the NIR region ascribed to intervalence charge transfer (IVCT) between the generated aminyl radical cations should be pointed out.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...