Quantitative prediction of the absorption maxima of azobenzene dyes from bond lengths and critical points in the electron density†
Abstract
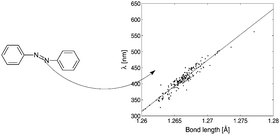
The relationship between the molecular electronic structure and the position of the absorption maxima in 191 azobenzene dyes has been studied by quantitative structure–property relations. A strong linearity is observed between the nitrogen–nitrogen bond length and the absorption wavelength with a squared correlation coefficient of 0.90. Bond lengths and properties of the critical points located on the electron density distribution are used to build partial least squares regression models for quantitative prediction of absorption wavelengths. Fifty of the azobenzene dyes were used as an external test set to evaluate the overall performance of the models. The simplest model where only the nitrogen–nitrogen bond length is used as a descriptor gives a root mean square error of prediction of 12.6 nm. When the value, laplacian and ellipticity of the electron density in all comparable bond critical points are used, the error of prediction is reduced to 5.4 nm. However, this model is less general and robust to prediction of novel molecular structures. It is demonstrated that the nitrogen–nitrogen bond in the azobenzene compounds relates to the colour of the dyes and in particular the nitrogen–nitrogen bond length plays a central role.

 Please wait while we load your content...
Please wait while we load your content...