Suppression of aqueous surface hydrolysis by monolayers of short chain organic amphiphiles
Abstract
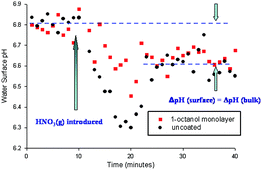
Aqueous aerosols and other water surfaces in the environment may be coated with organic films, which can give rise to significant effects on gas–solution transport and surface reactivity. We have used acridine as a molecular fluorescent pH probe to examine the hydration of nitric acid and ammonia at both the uncoated and the organic-coated air–water interface. For uncoated samples, a transient decrease in pH is observed at the interface upon introduction of nitric acid vapour, followed by a relaxation to a final pH which is lower than the initial value. This long-time final change in pH is also measured in bulk pH measurements. Solutions having monolayer and sub-monolayer films of 1-octanol do not display the transient, but do show the same long-time change in pH. The degree of suppression of the surface pH transient depends directly on the amount of octanol present at the surface. Hydrolysis of ammonia at the water surface is also indicated by a surface pH transient which is also suppressed when a monolayer of octanol is present at the surface. Monolayers of butanol and of uncompressed stearic acid at the surface show little difference from the clean interface. The results are related to the concentration of available water at the interface.

 Please wait while we load your content...
Please wait while we load your content...