Low temperature capture of open shell dipolar molecules by ions: the capture of rotationally selected NO(2Π1/2, j) by C+
Abstract
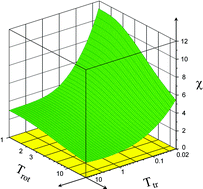
The low-energy capture of dipolar diatomic molecules in an open electronic state by ions is usually considered to be induced by the first-order charge–permanent dipole interaction with other terms of the long-range potential playing a minor role. If the molecular dipole moment is anomalously small (as is the case for slightly asymmetrical molecules), however, the situation changes, and the capture dynamics is strongly affected by higher orders of the charge–permanent dipole, charge–permanent quadrupole, and charge–induced dipole interactions. The interplay of different terms in the interaction potential manifests itself in complicated temperature dependence of the rotationally state-specific capture rate coefficients. These features of the capture are studied by way of example for NO(X 2Π1/2, j) + C+ collisions in the temperature range 10−2–20 K where the dynamics is adiabatic with respect to rotational and fine-structure transitions and sudden with respect to transitions between Λ doubling and hyperfine states. The theoretical rate coefficient, which depends on the translational and rotational temperature, agrees with the experimental one measured at Ttr = 0.6 K and Trot = 20 K.

 Please wait while we load your content...
Please wait while we load your content...