Reduced dimensionality quantum dynamics of Cl + CH4 → HCl + CH3 on an ab initio potential†
Abstract
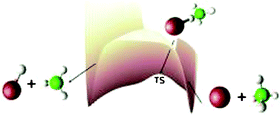
We study the reaction Cl + CH4
→ HCl + CH3 using a 2-D potential energy surface obtained by fitting a double Morse analytical function to high level (CCSD(T)/cc-pVTZ//MP2/cc-pVTZ)
ab initio data. Dynamics simulations are performed in hyperspherical coordinates with the close-coupled equations being solved using R-matrix propagation. Quantum contributions from spectator modes are included via a harmonic zero-point correction to the ab initio data prior to fitting the potential. This is the first time this method has been applied to a heavy–light–heavy reaction and the first time it has been used to study differential cross sections. We find thermal rate constants and state-to-state differential cross sections which are in good agreement with

 Please wait while we load your content...
Please wait while we load your content...