Abstract
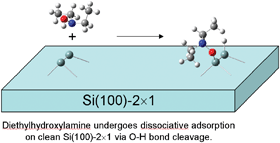
Incorporating diversity into structures constructed from the organic modification of silicon surfaces requires the use of molecules that contain multiple substituents of different types. In this work we examine the possible dissociation pathways of

 Please wait while we load your content...
Please wait while we load your content...