In order to establish the hydrogen-bond preference of an amide based N–H moiety faced with different C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or –OH hydrogen-bond acceptors, the crystal structures of several new co-crystals and salts were examined: 3-acetaminopyridine fumaric acid (2 : 1) 1, 4-(acetaminomethyl)pyridine fumaric acid (2 : 1) 2, 4-acetaminopyridine decanedioic (sebacic) acid (2 : 1) 3, 4-(acetaminomethyl)pyridine adipic acid (2 : 1) 4, 4-(acetaminomethyl)pyridine isophthalic acid (2 : 1) 5, 4-(acetaminomethyl)pyridinium 5-nitro-hydrogen isophthalate hydrate 6, 4-acetaminopyridinium hydrogenglutarate (1 : 1) 7. All co-crystals, 1–5, are constructed from an O–H(acid)⋯N(py) hydrogen bond and for the salts, 6–7, the primary synthon is the corresponding charge-assisted N–H+⋯−O interaction. The remaining N–H donor (on the amide moiety) shows a preference (4 out of 5) for the amide C
O or –OH hydrogen-bond acceptors, the crystal structures of several new co-crystals and salts were examined: 3-acetaminopyridine fumaric acid (2 : 1) 1, 4-(acetaminomethyl)pyridine fumaric acid (2 : 1) 2, 4-acetaminopyridine decanedioic (sebacic) acid (2 : 1) 3, 4-(acetaminomethyl)pyridine adipic acid (2 : 1) 4, 4-(acetaminomethyl)pyridine isophthalic acid (2 : 1) 5, 4-(acetaminomethyl)pyridinium 5-nitro-hydrogen isophthalate hydrate 6, 4-acetaminopyridinium hydrogenglutarate (1 : 1) 7. All co-crystals, 1–5, are constructed from an O–H(acid)⋯N(py) hydrogen bond and for the salts, 6–7, the primary synthon is the corresponding charge-assisted N–H+⋯−O interaction. The remaining N–H donor (on the amide moiety) shows a preference (4 out of 5) for the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O over the acid C
O over the acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or –OH hydrogen-bond acceptors, the crystal structures of several new co-crystals and salts were examined:
O or –OH hydrogen-bond acceptors, the crystal structures of several new co-crystals and salts were examined: ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O over the acid C
O over the acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.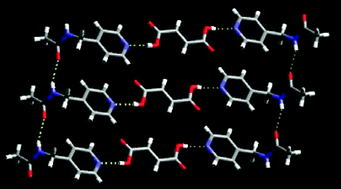

 Please wait while we load your content...
Please wait while we load your content...