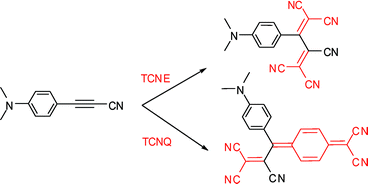
Donor-substituted 1,1,2,4,4-pentacyanobuta-1,3-dienes and a cyclohexa-2,5-diene-1,4-diylidene-expanded derivative were prepared by a [2 + 2] cycloaddition of tetracyanoethene (TCNE) or 7,7,8,8-tetracyanoquinodimethane (TCNQ) to anilino-substituted cyanoalkynes, followed by retro-electrocyclisation; they feature intense bathochromically-shifted intramolecular charge-transfer bands and undergo their first one-electron reductions at potentials similar to those reported for TCNE and TCNQ.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...