Insertion of benzyl isocyanide into a Zr–P bond and rearrangement. Atom-economical synthesis of a phosphaalkene†
Abstract
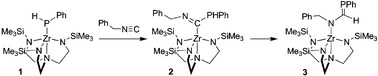
Reaction of (N3N)ZrPHPh (1, N3N = N(CH2CH2NSiMe3)33−) with PhCH2N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C affords the 1,1-insertion product (N3N)Zr[C(PHPh)
C affords the 1,1-insertion product (N3N)Zr[C(PHPh)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2Ph] (2), which thermally rearranges to the phosphaalkene-containing complex, (N3N)Zr[N(CH2Ph)C(H)
NCH2Ph] (2), which thermally rearranges to the phosphaalkene-containing complex, (N3N)Zr[N(CH2Ph)C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh] (3).
PPh] (3).

 Please wait while we load your content...
Please wait while we load your content...