Consecutive palladium-catalyzed Hiyama–Heck reactions in aqueous media under ligand-free conditions†
Abstract
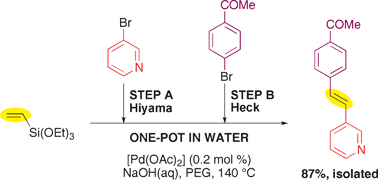
Symmetric and asymmetric (E)-1,2-diarylethenes are synthesized from aryl bromides by consecutive one-pot Hiyama–Heck reactions carried out in

 Please wait while we load your content...
Please wait while we load your content...