Ammonium lithocholate nanotubes: stability and copper metallization
Abstract
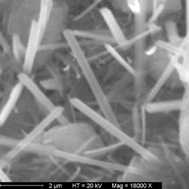
Ammonium lithocholate nanotubes (NH4LC) have been prepared in alkaline ammonia solutions and exhibited remarkable monodisperse cross-sectional dimensions (external diameter = 52 nm) as shown by cryo-transmission electron microscopy measurements. A classical electroless metallic replication method was used with a single poly(ethylene-imine) PEI layer coating the negatively charged NH4LC nanotubes. Short copper rods (external diameter ∼ 80 nm) were observed by scanning electron microscopy that corresponded to the original organic templates. The results obtained in acidic conditions are analyzed in terms of the lifetime of the self-assembled structures and formation of bundles of tubes. Dynamic light scattering measurements and optical observations show that the system in the presence of controlled amounts of hydrochloric acid is stable enough to account for a metallic replication in acidic conditions. An average apparent diffusion coefficient of the organic NH4LC assemblies is extracted (D ∼ 9.8 × 105 nm2 s−1) in homogeneous suspensions where bundles have been dispersed by the acidic additions.

 Please wait while we load your content...
Please wait while we load your content...