Diastereoselectivity of polar and radical couplings in electrophilic substitutions of rigid 2-lithio-N-methylpyrrolidines†
Abstract
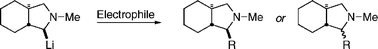
N-Methyl trans-fused perhydroisoindolines substituted with a tributylstannyl group in the 2-position have been prepared and used as precursors of the corresponding α-aminoorganolithiums. The steric course of the reactions of these and other conformationally rigid organolithiums with various electrophiles is summarized and compared with the steric course of the unsubstituted analogs. A mechanistic rationale for the steric course of electrophilic substitutions of these organolithiums is discussed. Pathways involving both polar electrophilic substitutions and radical couplings were observed with different electrophiles.

 Please wait while we load your content...
Please wait while we load your content...