Evidence for complexes of different stoichiometries between organic solvents and cyclodextrins†
Abstract
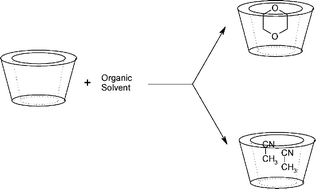
The influence of the organic solvent on the acid and basic hydrolysis of N-methyl-N-nitroso-p-toluenesulfonamide (MNTS) in the presence of α- and β-cyclodextrins has been studied. The observed rate constant was found to decrease through the formation of an unreactive complex between MNTS and the cyclodextrins. In the presence of dioxane, acetonitrile or DMSO, the inhibitory effect of β-CD decreased on increasing the proportion of organic cosolvent as a result of a competitive reaction involving the formation of an inclusion complex between β-CD and the cosolvent. The disparate size of the organic solvent molecules resulted in stoichiometric differences between the complexes; the β-CD–dioxane and β-CD–DMSO complexes were 1 : 1 whereas the β-CD–acetonitrile complex was 1 : 2. The basic and acid hydrolysis of MNTS in the presence of α-CD showed a different behavior; thus, the reaction gave both 1 : 1 and 2 : 1 α-CD–MNTS complexes, of which only the former was reactive. This result was due to the smaller cavity size of α-CD and the consequent decreased penetration of MNTS into the cavity in comparison to β-CD. The acid hydrolysis of MNTS in the presence of α-CD also revealed decreased penetration of MNTS into the cyclodextrin cavity, as evidenced by the bound substrate undergoing acid hydrolysis. In addition, the acid hydrolysis of MNTS in the presence of acetonitrile containing α-CD gave 1 : 1 α-CD–acetonitrile inclusion complexes, which is consistent with a both a reduced cavity size and previously reported data.

 Please wait while we load your content...
Please wait while we load your content...