Inclusion complexes of EMPO derivatives with 2,6-di-O-methyl-β-cyclodextrin: synthesis, NMR and EPR investigations for enhanced superoxide detection†
Abstract
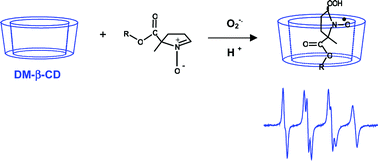
The free radical trapping properties of eight 5-alkoxycarbonyl-5-methyl-1-pyrroline N-oxide (EMPO) type nitrones and those of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were evaluated for trapping of superoxide anion radicals in the presence of 2,6-di-O-methyl-β-cyclodextrin (DM-β-CD). 1H-NMR titrations were performed to determine both stoichiometries and binding constants for the diamagnetic nitrone–DM-β-CD equilibria. EPR titrations were then performed and analyzed using a two-dimensional EPR simulation program affording 1 : 1 and 1 : 2 stoichiometries for the nitroxide spin adducts with DM-β-CD and the associated binding constants after spin trapping. The nitroxide spin adducts associate more strongly with DM-β-CD than the nitrones. The ability of the nitrones to trap superoxide, the enhancement of the EPR signal intensity and the supramolecular protection by DM-β-CD against sodium L-ascorbate

 Please wait while we load your content...
Please wait while we load your content...