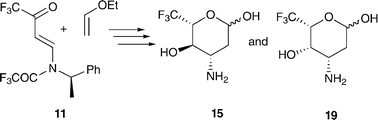
A short synthesis of 6,6,6-trifluoro-L-acosamine 15 and 6,6,6-trifluoro-L-daunosamine 19 has been accomplished. The pyranose ring system of these carbohydrate analogues was formed by a hetero-Diels–Alder reaction of vinylogous imide 11 and ethyl vinyl ether which gave adduct 12a in 40% yield. Hydroboration gave 13 and subsequent hydrogenolytic removal of the (R)-2-phenylethyl chiral auxiliary gave ethyl 6,6,6-trifluoro-L-acosaminide 14. Acid hydrolysis furnished target 15. Glycoside 13 was N-trifluoroacetylated to give 16, the structure was confirmed by single crystal X-ray diffraction. The C-4 stereochemistry of 16 was inverted by Swern oxidation of the 4-OH group, and subsequent borohydride reduction to give 17. Hydrogenolytic removal of the auxiliary gave ethyl-6,6,6-trifluoro-L-daunosaminide 18. Acid hydrolysis provided 19.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...