Selective product amplification of thymine photodimer by recognition-directed supramolecular assistance
Abstract
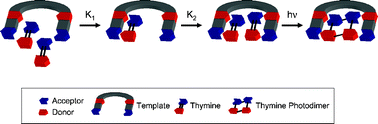
Two symmetric ditopic supramolecular templates (1 and 2) each presenting two hydrogen bonding recognition subunits were synthesized. Each such subunit comprises the same donor and acceptor pattern, capable of binding a substrate molecule with complementary hydrogen bonding groups to form a supramolecular complex. Substrate molecules, such as

 Please wait while we load your content...
Please wait while we load your content...