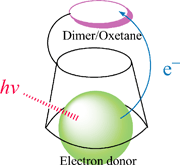
Two modified β-cyclodextrins (β-CDs) with a thymine dimer and a thymine oxetane adduct respectively, TD-CD and Ox-CD, have been prepared, and utilized to bind an electron-rich chromophore, indole or N,N-dimethylaniline (DMA), to form a supramolecular complex. We have examined the photosensitized splitting of the dimer/oxetane unit in TD-CD/Ox-CD by indole or DMA via an electron-transfer pathway, and observed high splitting efficiencies of the dimer/oxetane unit. On the basis of measurements of fluorescence spectra and splitting quantum yields, it is suggested that the splitting reaction occurs in a supramolecular complex by an inclusion interaction between the modified β-CDs and DMA or indole. The back electron transfer, which leads low splitting efficiencies for the covalently-linked chromophore–dimer/oxetane compounds, is suppressed in the non-covalently-bound complex, and the mechanism has been discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...