Enhanced stereocontrol in Diels–Alder reactions of chiral dienols†
Abstract
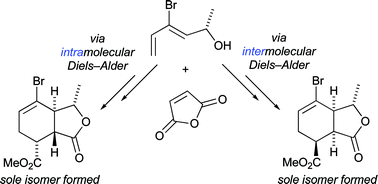
This combined experimental–computational investigation demonstrates that the presence of a removable bromine substituent on a diene leads to complete π-diastereofacial and endo/exo stereoselection in both intermolecular and intramolecular Diels–Alder reactions. The influence of the bromine upon stereoselectivity is dramatic: the cycloaddition of nonbrominated precursor 18EEEEEE, for example, gives four diastereomeric products in a 55∶13∶16∶16 ratio; the bromine-containing analogue gives one stereoisomer within the limits of detection. The examination of B3LYP/6-31+G(d) transition structures allows an interpretation of these experimental findings. A method for the completely stereoselective synthesis of complimentary diastereomeric products (30ZZZZZZ and 31ZZZZ) from the same simple starting materials (28 and 2) is reported. Discrepancies between calculation and experiment in an earlier investigation into the Diels–Alder reaction are explained.

 Please wait while we load your content...
Please wait while we load your content...