A computational exploration of some transnitrosation and thiolation reactions involving CH3SNO, CH3ONO and CH3NHNO
Abstract
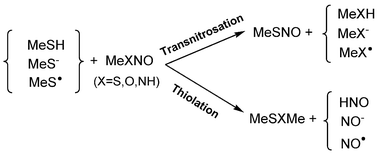
Nitric oxide (NO) is a biologically active species and its carrier molecules RXNO (X = S, O, NH) have drawn significant attention recently. In the present work, the CBS-QB3 level of theory was used to study the transnitrosation and thiolation reaction between MeXNO (X = S, O, and NH) molecules and three reactive forms of the methanethiol: the neutral molecule, MeSH, the anion, MeS−, and the radical, MeS˙. The transnitrosation and thiolation reactions between MeXNO and MeSH have the highest barriers, both with and without a molecule of

 Please wait while we load your content...
Please wait while we load your content...