Microtiter plate based chemistry and in situ screening: a useful approach for rapid inhibitor discovery
Abstract
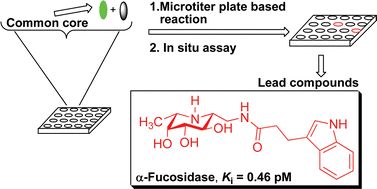
The use of libraries extracted from nature or constructed by combinatorial chemistry, have been widely appreciated in the drug discovery area. In this perspective, we present our contribution to the field of enzyme inhibitor discovery using a useful approach that allows diversification of a common core in a microtiter plate followed by in situ screening. Our method relies on an organic reaction that is highly selective, high yielding, amenable to the microscale and preferably can be performed in water. The core can be a designed molecule based on the structural and mechanistic information of the target, a compound with a weak binding affinity, or a natural product. Several reactions were found useful for this approach and were applied to the rapid discovery of potent inhibitors of representative enzymes.

 Please wait while we load your content...
Please wait while we load your content...