Diastereoselective cyclization of a dithienylethene switch through single crystal confinement†
Abstract
Upon UV irradiation of the hydrogen-bond confined crystal state of a dithienylhexafluorocyclopentene with (R)-N-phenylethylamide substituents, the photochemical cyclization reaction proceeds diastereoselectively to form the coloured, closed-ring isomer with 97% de.
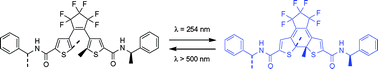

 Please wait while we load your content...
Please wait while we load your content...