Non-identical electronic characters of the internucleotidic phosphates in RNA modulate the chemical reactivity of the phosphodiester bonds†‡
Abstract
We here show that the electronic properties and the chemical reactivities of the internucleotidic phosphates in the heptameric ssRNAs are dissimilar in a sequence-specific manner because of their non-identical microenvironments, in contrast with the corresponding isosequential ssDNAs. This has been evidenced by monitoring the δ H8(
H8(![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) ) shifts upon pH-dependent ionization (pKa1) of the central 9-guaninyl (
) shifts upon pH-dependent ionization (pKa1) of the central 9-guaninyl (![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) ) to the 9-guanylate ion (G−), and its electrostatic effect on each of the internucleotidic phosphate anions, as measured from the resultant δ
) to the 9-guanylate ion (G−), and its electrostatic effect on each of the internucleotidic phosphate anions, as measured from the resultant δ 31P shifts (pKa2) in the isosequential heptameric ssRNAs vis-à-vis ssDNAs: [d/r(5′-Cp1Ap2Q1p3
31P shifts (pKa2) in the isosequential heptameric ssRNAs vis-à-vis ssDNAs: [d/r(5′-Cp1Ap2Q1p3![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) p4Q2p5Ap6C-3′): Q1 = Q2 = A (5a/5b) or C (8a/8b), Q1 = A, Q2 = C (6a/6b), Q1 = C, Q2 = A (7a/7b)]. These oligos with single ionizable
p4Q2p5Ap6C-3′): Q1 = Q2 = A (5a/5b) or C (8a/8b), Q1 = A, Q2 = C (6a/6b), Q1 = C, Q2 = A (7a/7b)]. These oligos with single ionizable ![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) in the centre are chosen because of the fact that the pseudoaromatic character of
in the centre are chosen because of the fact that the pseudoaromatic character of ![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) can be easily modulated in a pH-dependent manner by its transformation to G− (the 2′-OH to 2-O− ionization effect is not detectable below pH 11.6 as evident from the N1-Me-
can be easily modulated in a pH-dependent manner by its transformation to G− (the 2′-OH to 2-O− ionization effect is not detectable below pH 11.6 as evident from the N1-Me-![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) analog), thereby modulating/titrating the nature of the electrostatic interactions of
analog), thereby modulating/titrating the nature of the electrostatic interactions of ![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) to G− with the phosphates, which therefore constitute simple models to interrogate how the variable pseudoaromatic characters of nucleobases under different sequence context (J. Am. Chem. Soc., 2004, 126, 8674–8681) can actually influence the reactivity of the internucleotide phosphates as a result of modulation of sequence context-specific electrostatic interactions. In order to better understand the impact of the electrostatic effect of the
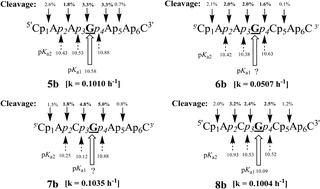
to G− with the phosphates, which therefore constitute simple models to interrogate how the variable pseudoaromatic characters of nucleobases under different sequence context (J. Am. Chem. Soc., 2004, 126, 8674–8681) can actually influence the reactivity of the internucleotide phosphates as a result of modulation of sequence context-specific electrostatic interactions. In order to better understand the impact of the electrostatic effect of the ![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) to G− on the tunability of the electronic character of internucleotidic phosphates in the heptameric ssRNAs 5b, 6b, 7b and 8b, we have also performed their alkaline hydrolysis at pH 12.5 at 20 °C, and have identified the preferences of the cleavage sites at various phosphates, which are p2, p3 and p4 (
to G− on the tunability of the electronic character of internucleotidic phosphates in the heptameric ssRNAs 5b, 6b, 7b and 8b, we have also performed their alkaline hydrolysis at pH 12.5 at 20 °C, and have identified the preferences of the cleavage sites at various phosphates, which are p2, p3 and p4 (![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) heptameric ssRNA sequences 5c, 7c and 8c under identical conditions in order to establish the role of the electrostatic effect of the 9-guanylate ion (and the 2′-OH to 2-O− ionization) on the internucleotidic phosphate. It turned out that the relative alkaline hydrolysis rate at those particular phosphates (p2, p3 and p4) in the N1-Me-
heptameric ssRNA sequences 5c, 7c and 8c under identical conditions in order to establish the role of the electrostatic effect of the 9-guanylate ion (and the 2′-OH to 2-O− ionization) on the internucleotidic phosphate. It turned out that the relative alkaline hydrolysis rate at those particular phosphates (p2, p3 and p4) in the N1-Me-![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) heptamers was reduced from 16–78% compared to those in the native counterparts [
heptamers was reduced from 16–78% compared to those in the native counterparts [

 Please wait while we load your content...
Please wait while we load your content...