Incorporation of deuterium-labelled analogs of isopentenyl diphosphate for the elucidation of the stereochemistry of rubber biosynthesis
Abstract
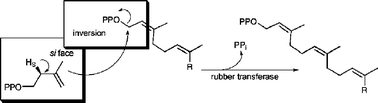
A series of six deuterium-labelled analogs of isopententyl diphosphate (IPP) was prepared to investigate the detailed stereochemical course of addition of C5 units during rubber biosynthesis in Hevea brasiliensis and Parthenium argentatum. These analogs were incorporated into the cis-polyisoprene chain by rubber transferase in

 Please wait while we load your content...
Please wait while we load your content...