Conformational arm-wrestling: battles for stereochemical control in benzamides bearing matched and mismatched chiral 2- and 6-substituents
Abstract
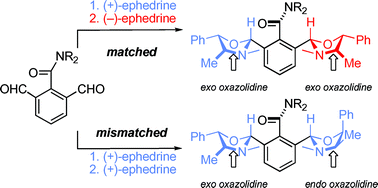
The orientation of a tertiary amide group adjacent to an aromatic ring may be governed by the stereochemistry of an adjacent chiral substituent. With a chiral substituent in both ortho positions, matched/mismatched pairs of isomers result. Evidence for matched stereochemistry is provided by the clean NMR spectra of single conformers, while mismatching gives poor or unexpected selectivities in the formation of chiral substituents, or mixtures of

 Please wait while we load your content...
Please wait while we load your content...