Aromatic annulation strategy for naphthalenes fused at 1,2- and 3,4-positions with two heterocycles†
Abstract
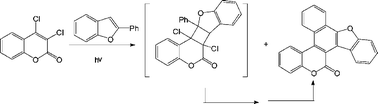
An efficient regioselective naphthoannulation strategy able to fuse a newly formed naphthalene ring at its 1,2- and 3,4- positions to two different heterocycles has been developed.

 Please wait while we load your content...
Please wait while we load your content...