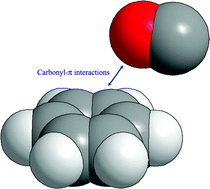
In this work we present some experimental evidence of the existence of carbonyl–π electron cloud interactions. Such interactions are analogous to anion–π interactions, which have been predicted to be energetically favourable in the case of electron deficient aromatic rings. UV-Visible spectroscopy and cyclic voltammetry results obtained for 9,10-anthraquinone, 1,1′-bis-9,10-anthraquinone, poly(9,10-anthraquinone-1,4-diyl) and other 1,4-diaryl substituted anthraquinone derivatives are described. It was found that the steric hindrance occurring between the carbonyl groups and the adjacent aromatic substituent forces the plane of the anthraquinone moiety and that of the aromatic substituent to adopt a nearly orthogonal conformation, resulting in relatively strong carbonyl–π interactions that affect both the UV-Vis absorption spectrum and the reduction potential of the compound. Moreover, in the case of thiophene substituted derivatives, the torsion angle between the anthraquinone moiety and its aromatic substituent is smaller and therefore carbonyl–π interaction effects are not observed in these compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...