Synthesis and photoresponse of a fullerene–bis(styryl)benzene dyad†
Abstract
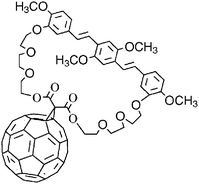
As part of our investigations into single-component organic solar cells, a symmetrical bis(styryl)benzene–fullerene dyad has been synthesized. An oxyethylene macrocyclic spacer, attached to meta-positions of side phenyl groups of bis(styryl)benzene, was linked to [60]fullerene through a synthesis based on the nucleophilic Bingel cyclopropanation of the fullerenes. The dyad structure was confirmed by 1H, 13C and HR-FAB mass spectra. The UV-Vis spectrum of the dyad is the superimposition of those of appropriate model systems, indicating that ground-state electronic interactions between the constituent chromophores, in solution, are negligible, in line also with the electrochemical results. Molecular modelling studies and NMR data suggest that the bis(styryl)benzene moves back and forth at room temperature from one side of the fullerene to the other. Interestingly, the overall power conversion efficiency (η) calculated for solar cells made of the bis(styryl)benzene–fullerene dyad was 0.022% under a white-light irradiation power of 86 mW cm−2. This is, to our knowledge, the highest value reported, under high-level irradiation conditions, for solar cells based on dyads in which a fullerene is tethered to a phenylenevinylene structure.

 Please wait while we load your content...
Please wait while we load your content...