Experimental determination of the conformational free energies (A values) of fluorinated substituents in cyclohexane by dynamic 19F NMR spectroscopy. Part 2.† Extension to fluoromethyl, difluoromethyl, pentafluoroethyl, trifluoromethylthio and trifluoromethoxy groups‡
Abstract
The synthesis of monosubstituted and 1,4-substituted cyclohexanes bearing one of the title groups is described. The conformational analysis of these compounds was studied by 19F NMR spectroscopy at various temperatures. Chemical shifts for each conformer above the coalescence temperature were obtained by binomial regression from low temperature values, allowing the high precision determination of the equilibrium constants, and the corresponding thermodynamic parameters (ΔG°, ΔH°, ΔS°) of the fluorinated substituents. For A values (−ΔG°298K), the following averaged data were obtained: 1.59 (CFH2), 1.85 (CF2H), 2.67 (C2F5), 0.79 (OCF3) and 1.18 (SCF3) [in kcal mol−1].
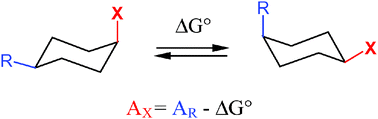

 Please wait while we load your content...
Please wait while we load your content...