Hydrophilic interior between hydrophobic regions in inverse bilayer structures of cation–1,1′-binaphthalene-2,2′-diyl phosphate salts†
Abstract
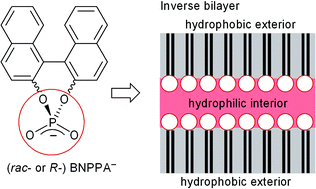
A series of 1,1′-binaphthalene-2,2′-diyl phosphate (BNPPA−) salts have been synthesized. Their crystal packings show a separation of the hydrophobic naphthyl and hydrophilic (RO)2PO2− phosphate/cation/solvate regions. Hydrogen bonding in the latter is the driving force for “inverse bilayer” formation, with a hydrophilic interior exposing the hydrophobic binaphthyl groups to the exterior. Stacking of the inverse bilayers occurs less through π–π and more through CH⋯π interactions between the naphthyl groups, which correlates with the formation of thin crystal plates along the stacking direction. Cations used with R- or rac-BNPPA− are protonated isonicotin-1-ium amide (1), isonicotin-1-ium acid (2), guanidinium (3), the metal complexes trans-tetraammine-dimethanol-copper(II) (4), trans-diaqua-tetramethanol-copper(II) (5) and cis-diaqua-bis(ethylene diamine)-nickel(II) (6). Crystallization occurs with inclusion of water and methanol solvent molecules, except in 2. Starting from R-BNPPA, inversion takes place with calcium acetate to give 1 as the racemate. 2 is crystallized as the R-BNPPA salt. The inversion-symmetrical complex trans-[Cu(H2O)2(CH3OH)4]2+ in 5 has Cu–OH2 bond lengths of 1.937(4) Å, and Cu–O(methanol) of 2.112(4) and 2.167(4) Å, corresponding to a compressed tetragonal geometry.

 Please wait while we load your content...
Please wait while we load your content...