Myriaporone 3/4 structure–activity relationship studies define a pharmacophore targeting eukaryotic protein synthesis†
Abstract
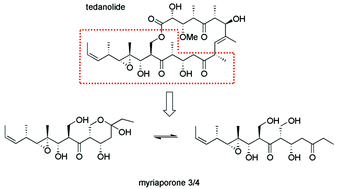
Myriaporones are naturally occurring compounds which structurally resemble the southern hemisphere of the tedanolide family of
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...