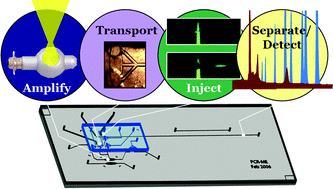
Poly(dimethylsiloxane) (PDMS) membrane valves were utilized for diaphragm pumping on a PDMS-glass hybrid microdevice in order to couple infrared-mediated DNA amplification with electrophoretic separation of the products in a single device. Specific amplification products created during non-contact, infrared (IR) mediated polymerase chain reaction (PCR) were injected via chip-based diaphragm pumping into an electrophoretic separation channel. Channel dimensions were designed for injection plug shaping via preferential flow paths, which aided in minimizing the plug widths. Unbiased injection of sample could be achieved in as little as 190 ms, decreasing the time required with electrokinetic injection by two orders of magnitude. Additionally, sample stacking was promoted using laminar or biased-laminar loading to co-inject either water or low ionic strength DNA marker solution along with the PCR-amplified sample. Complete baseline resolution (Res = 2.11) of the 80- and 102-bp fragments of pUC-18 DNA marker solution was achieved, with partially resolved 257- and 267-bp fragments (Res = 0.56), in a separation channel having an effective length of only 3.0 cm. This resolution was deemed adequate for many PCR amplicon separations, with the added advantage of short separation time—typically complete in <120 s. Decreasing the amount of glass surrounding the PCR chamber reduced the DNA amplification time, yielding a further enhancement in analysis speed, with heating and cooling rates as high as 13.4 and −6.4 °C s−1, respectively. With the time requirements greatly reduced for each step, it was possible to seamlessly couple IR-mediated amplification, sample injection, and separation/detection of a 278-bp fragment from the invA gene of <1000 starting copies of Salmonella typhimurium DNA in ∼12 min on a single device, representing the fastest PCR-ME integration achieved to date.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...