A theoretical and experimental study of the distorted pyrochlore Bi2Sn2O7
Abstract
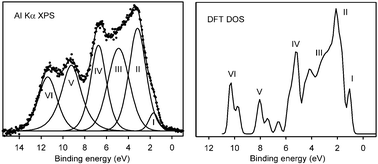
The pyrochlore based bismuth stannate, Bi2Sn2O7, is a material with important applications in catalysis and gas sensing. The thermodynamically stable α phase has 352 atoms in the unit cell and is one of the most complex

 Please wait while we load your content...
Please wait while we load your content...