Vitreous samples were prepared in the (100 −
x) NaPO3–x WO3 (0 ≤
x
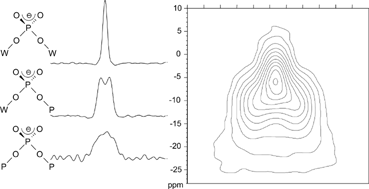
≤ 70) glass forming system using conventional melting–quenching methods. The structural evolution of the vitreous network was monitored as a function of composition by thermal analysis, Raman spectroscopy and high resolution one- and two-dimensional 31P solid state NMR. Addition of WO3 to the NaPO3 glass melt leads to a pronounced increase in the glass transition temperatures, suggesting a significant increase in network connectivity. At the same time Raman spectra indicate that up to about 30 mol% WO3 the tungsten atoms are linked to some non-bridging oxygen atoms (W–O− or W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonded species), suggesting that the network modifier sodium oxide is shared to some extent between both network formers. W–O–W bond formation occurs only at WO3 contents exceeding 30 mol%. 31P magic angle spinning (MAS)-NMR spectra, supported by two-dimensional J-resolved spectroscopy, allow a clear distinction between species having two, one, and zero P–O–P linkages. The possible formation of some anionic tungsten sites suggested from the Raman data implies an average increase in the degree of polymerization for the phosphorus species, which would result in diminished 31P/23Na interactions. This prediction is indeed confirmed by 31P{23Na} and 23Na{31P} rotational echo double resonance (REDOR) NMR results, which indicate that successive addition of WO3 to NaPO3 glass significantly diminishes the strength of phosphorus–sodium dipole–dipole couplings.
O bonded species), suggesting that the network modifier sodium oxide is shared to some extent between both network formers. W–O–W bond formation occurs only at WO3 contents exceeding 30 mol%. 31P magic angle spinning (MAS)-NMR spectra, supported by two-dimensional J-resolved spectroscopy, allow a clear distinction between species having two, one, and zero P–O–P linkages. The possible formation of some anionic tungsten sites suggested from the Raman data implies an average increase in the degree of polymerization for the phosphorus species, which would result in diminished 31P/23Na interactions. This prediction is indeed confirmed by 31P{23Na} and 23Na{31P} rotational echo double resonance (REDOR) NMR results, which indicate that successive addition of WO3 to NaPO3 glass significantly diminishes the strength of phosphorus–sodium dipole–dipole couplings.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonded species), suggesting that the network modifier
O bonded species), suggesting that the network modifier 

 Please wait while we load your content...
Please wait while we load your content...