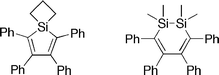
We present an experimental and density functional theory comparison of the geometric structure, electronic characteristics and optical properties of 1,1-dimethyl-2,3,4,5-tetraphenylsilole (I), 1,1-(propane-1,3-diyl)-2,3,4,5-tetraphenylsilole (II), 1,1-dimethoxy-2,3,4,5-tetraphenylsilole (III) and 1,1,2,2-tetramethyl-3,4,5,6-tetraphenyl-1,2-disila-3,5-cyclohexadiene (IV). The molecular structures of II–IV have been determined using X-ray crystallography and are compared to the previously reported structure of I. Many characteristics of IV are significantly different from those of I–III, due to the presence of the additional silicon atom. In contrast to I–III, which have planar central rings, IV has a non-planar ring conformation due to the presence of two approximately tetrahedrally coordinated silicon atoms. Cyclic voltammetry suggests that I–III are reduced at similar potentials to that for tris(8-hydroquinolinato)aluminium and that IV is more readily oxidizable than I–III. Additionally, while IV is non-emissive in both solution and the solid state, the three siloles are fluorescent in the solid state. For the four compounds, we have calculated the intramolecular reorganization energies for electron- and hole-transfer reactions, ionization potentials and electron affinities.

 Please wait while we load your content...
Please wait while we load your content...