The effect of carborane, bicyclo[2.2.2]octane and benzene on mesogenic and dielectric properties of laterally fluorinated three-ring mesogens†
Abstract
Six series of structurally similar compounds containing 12- and 10-vertex p-carborane (A and B), 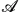 (the slope = 14.8 °C Å−1 and R2 = 0.997). Compounds in series 2 (X = F) were used as low concentration additives to a nematic host, 6-CHBT. Dielectric parameters were extrapolated to pure additives and analyzed using the Maier–Meier equation. The Kirkwood factors g and apparent order parameters Sapp that are required to reproduce the extrapolated dielectric values follow the trend in the size of ring
(the slope = 14.8 °C Å−1 and R2 = 0.997). Compounds in series 2 (X = F) were used as low concentration additives to a nematic host, 6-CHBT. Dielectric parameters were extrapolated to pure additives and analyzed using the Maier–Meier equation. The Kirkwood factors g and apparent order parameters Sapp that are required to reproduce the extrapolated dielectric values follow the trend in the size of ring 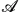 . The smallest g (0.47) and the largest Sapp (6.3) are obtained for
. The smallest g (0.47) and the largest Sapp (6.3) are obtained for 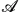 .
.
![Graphical abstract: The effect of carborane, bicyclo[2.2.2]octane and benzene on mesogenic and dielectric properties of laterally fluorinated three-ring mesogens](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B600068A&imageInfo.ImageIdentifier.Year=2006)

 Please wait while we load your content...
Please wait while we load your content...