LiTixZr2−x(PO4)3 (0 ≤
x
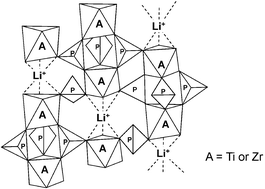
≤ 2) (LiTZP(x)) were synthesized by heating stoichiometric mixtures of lithium carbonate, zirconyl nitrate, titanium oxide and phosphoric acid at 950 °C for 10 hours. Ion exchangers in the hydrogen form (HTZP(x)) were prepared by the acid treatment of LiTZP(x) and their cation exchange properties were investigated with group 1 and group 2 metal ions. While the topotactic ion exchange from LiTZP(x) to HTZP(x) occurred for 0 ≤
x
≤ 1.5, the main phase of HTZP(2.0) was semicrystalline layered Ti2O3(H2PO4)2·2H2O. All the ion exchangers obtained were not very stable and partially decomposed under basic conditions. HTZP(x) (0 ≤
x
≤ 1.5) showed high selectivity towards the lithium ion from among group 1 metal ions, whereas HTZP(2.0) showed no specific affinity towards any group 1 metal ions. The H+/Li+ ion exchange equilibrium in acidic conditions at 25 °C was attained within 7 days for HTZP(0.0), in 1 day for HTZP(0.5), HTZP(1.0) and HTZP(1.3) and within 1 min for HTZP(2.0). The H+/Li+ ion exchange rate thus increased with increasing x. The exceptionally high ion exchange rate on HTZP(2.0) could be ascribed to the layered structure of the main phase of HTZP(2.0). All the ion exchangers synthesized preferentially took up the lighter isotope of lithium (6Li) over the heavier counterpart (7Li). The maximum value of the lithium isotopic separation factor (S) was 1.044 obtained at x = 1.0 to 1.3. This indicated that the size and the nature of ion exchange sites was a determining factor of S, which depended on the differences in the ionic radius and electronegativity of titanium(IV) and zirconium(IV).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...