Hydrophobic induced supramolecular self-organization of tetrahedral units based on van der Waals interactions†
Abstract
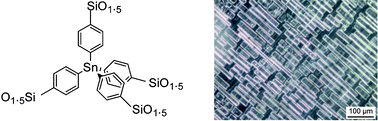
Nanostructured hybrid materials were prepared by sol–gel hydrolysis–polycondensation of tetrakis(4-trisisopropoxysilylphenyl)germane PGe and tetrakis(4-trisisopropoxysilylphenyl) tin PSn which present a tetrahedral germanium or tin central atom. Both precursors presented twelve directions for solid formation oriented regularly on the four directions of the tetrahedron. Their organization was evaluated by X-ray diffraction studies (nanometric scale) and birefringence measurements (micrometric scale). It has been shown that the organization of these solids was influenced by the experimental parameters. The intensities of birefringence lay in the same range (1.6 × 10−3 to 2.1 × 10−3) for all the gels XGe and XSn. Regular parallel rectangular chunks were observed in all cases. The orientation of the optical axis was found perpendicular to the edges of the chunks in all cases. The existence of such optical axes regularly oriented towards the edges of the chunks corresponded to an anisotropic organization at the micrometric scale. The results presented here strengthen the fact that the auto-organization observed in nanostructured organic–inorganic hybrid materials occurs during the formation of Si–O–Si bonds, which transforms intermolecular interactions into intramolecular ones corresponding to the weak van der Waals-type interactions existing between the organic units. The role of hydrophilic–hydrophobic interactions is discussed.

 Please wait while we load your content...
Please wait while we load your content...