Efficient regioselective acylation of 1-β-d-arabinofuranosylcytosine catalyzed by lipase in ionic liquid containing systems
Abstract
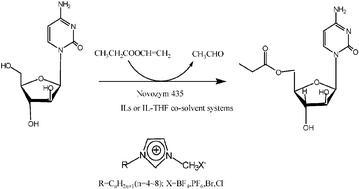
Seven ionic liquids (ILs) were tested for use in the regioselective acylation of 1-β-D-arabinofuranosylcytosine (ara-C) by vinyl propionate, catalyzed by immobilized Candida antarctica lipase B. The results demonstrated that the nature of both the cations and the anions of ILs had a significant effect on the initial rate and the substrate conversion, but little effect on the regioselectivity of the reaction. The lipase displayed enhanced activity toward ara-C when the alkyl chain of CnMIm·BF4 increased in length (n = 4–8) and no acylation reaction occurred in C4MIm·Cl or C4MIm·Br. To further enhance the initial rate and substrate conversion, co-solvent mixtures of ILs and organic solvents were investigated. Among various IL-containing systems examined, 10% (v/v) C4MIm·PF6–tetrahydrofuran gave the highest initial rate and substrate conversion. In this reaction medium, the optimal water activity, vinyl propionate/ara-C molar ratio, temperature and shaking rate were 0.07, 15 ∶ 1 (mol/mol), 60 °C and 250 rpm, respectively. Under these conditions, the initial rate, substrate conversion and the regioselectivity were 94.0 mM h−1, 98.5% and 99%, respectively. An additional comparative study demonstrated that the enzymatic acylation proceeded with very similar initial rate, substrate conversion, regioselectivity and activation energy whether the reaction medium was 10% (v/v) C4MIm·PF6–tetrahydrofuran or 28% (v/v) hexane–pyridine (the best organic solvent mixture for the reaction). However, the lipase exhibited a much higher stability in the IL-containing system, which may also have environmental advantages. The product of the lipase-catalysed reaction was characterized by NMR, FT-IR spectroscopy and was shown to be the 5′-O-monoester of ara-C.

 Please wait while we load your content...
Please wait while we load your content...